Baking soda and baking powder serve different leavening roles in cookies, with baking soda requiring an acidic ingredient to activate and produce carbon dioxide for rise. Baking powder contains both an acid and base, enabling it to release gas twice during baking for a lighter texture. Choosing between the two affects cookie spread and texture, as baking soda yields chewier results while baking powder creates fluffier cookies.
Table of Comparison
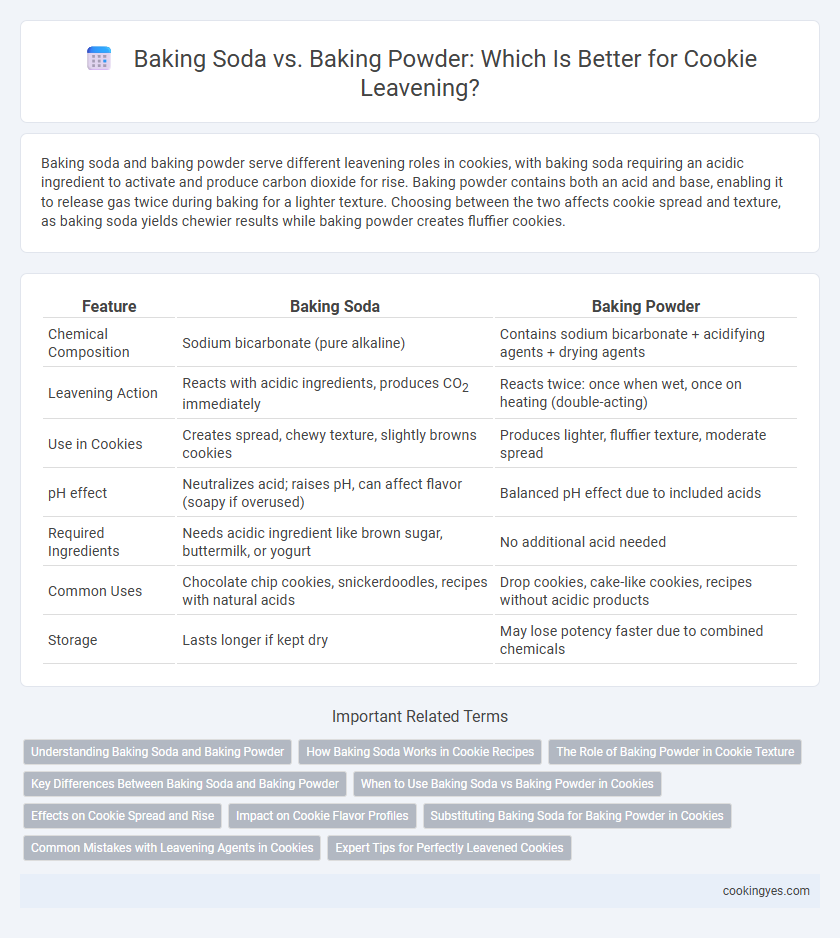
| Feature | Baking Soda | Baking Powder |
|---|---|---|
| Chemical Composition | Sodium bicarbonate (pure alkaline) | Contains sodium bicarbonate + acidifying agents + drying agents |
| Leavening Action | Reacts with acidic ingredients, produces CO2 immediately | Reacts twice: once when wet, once on heating (double-acting) |
| Use in Cookies | Creates spread, chewy texture, slightly browns cookies | Produces lighter, fluffier texture, moderate spread |
| pH effect | Neutralizes acid; raises pH, can affect flavor (soapy if overused) | Balanced pH effect due to included acids |
| Required Ingredients | Needs acidic ingredient like brown sugar, buttermilk, or yogurt | No additional acid needed |
| Common Uses | Chocolate chip cookies, snickerdoodles, recipes with natural acids | Drop cookies, cake-like cookies, recipes without acidic products |
| Storage | Lasts longer if kept dry | May lose potency faster due to combined chemicals |
Understanding Baking Soda and Baking Powder
Baking soda and baking powder are essential leavening agents in cookie baking, each serving different chemical roles. Baking soda is pure sodium bicarbonate that requires an acidic ingredient, like brown sugar or buttermilk, to activate and produce carbon dioxide gas for dough rise. Baking powder contains sodium bicarbonate and acidifying agents, allowing it to react twice--once when moistened and again when heated--making it ideal for recipes without acidic components.
How Baking Soda Works in Cookie Recipes
Baking soda acts as a leavening agent by reacting with acidic ingredients in cookie recipes, producing carbon dioxide gas that helps the dough rise and creates a tender texture. Its effectiveness depends on the presence of acids like brown sugar, buttermilk, or molasses to activate the chemical reaction. Proper balance of baking soda is crucial; too much can cause a metallic taste, while too little results in dense cookies with minimal leavening.
The Role of Baking Powder in Cookie Texture
Baking powder acts as a chemical leavening agent that releases carbon dioxide gas when moistened and heated, creating a lighter, airier cookie texture. Unlike baking soda, baking powder contains both an acid and a base, allowing it to produce leavening without additional acidic ingredients in the dough. This results in cookies that are fluffier and tender, enhancing overall softness and rise.
Key Differences Between Baking Soda and Baking Powder
Baking soda and baking powder are both leavening agents used in cookie recipes but function differently due to their chemical compositions. Baking soda, or sodium bicarbonate, requires an acidic ingredient like buttermilk or lemon juice to activate and produce carbon dioxide for leavening, resulting in a faster rise and a crispier texture. Baking powder contains both an acid and a base, allowing it to leaven dough without additional acidic components, typically creating a softer, fluffier cookie with a more neutral taste.
When to Use Baking Soda vs Baking Powder in Cookies
Baking soda is ideal for cookies containing acidic ingredients like brown sugar, yogurt, or lemon juice, as it reacts immediately to create a tender, spread-out texture. Baking powder, containing both an acid and a base, works best in recipes without acidic components to provide a slower, sustained rise, resulting in thicker, fluffier cookies. For recipes with balanced acidity, a combination of baking soda and baking powder can optimize leavening, texture, and flavor.
Effects on Cookie Spread and Rise
Baking soda creates a higher pH environment that promotes browning and spreads cookies more due to faster leavening during baking. Baking powder contains both an acid and base, producing CO2 gas in two stages for a balanced rise and thicker, puffier cookies. Using baking soda typically results in thinner, crisper edges, while baking powder yields a taller, softer cookie texture.
Impact on Cookie Flavor Profiles
Baking soda produces a subtle metallic or alkaline taste that can enhance the caramelization and browning of cookies, contributing to a deeper, more complex flavor profile. Baking powder offers a more neutral taste with a balanced leavening effect, resulting in softer, fluffier cookies with a milder flavor. The choice between baking soda and baking powder significantly influences cookie texture and flavor intensity, with baking soda favoring sharper, tangy notes while baking powder supports a tender, slightly sweet finish.
Substituting Baking Soda for Baking Powder in Cookies
Substituting baking soda for baking powder in cookies requires adjusting the acidic ingredients because baking soda needs an acid to activate its leavening process, whereas baking powder contains both an acid and a base. Using baking soda alone without additional acidic components like cream of tartar, yogurt, or lemon juice may result in flat, dense cookies due to insufficient rise. Proper measurement is crucial; generally, one teaspoon of baking powder can be replaced with 1/4 teaspoon baking soda plus an appropriate acid to maintain cookie texture and lift.
Common Mistakes with Leavening Agents in Cookies
Using baking soda instead of baking powder in cookie recipes often leads to incorrect rise and texture due to differences in their chemical composition; baking soda requires an acidic ingredient to activate, while baking powder contains its own acid. A common mistake is overusing baking powder, causing cookies to become cakey or develop a bitter taste. Ensuring the correct type and amount of leavening agent is crucial for achieving the intended cookie crumb and spread.
Expert Tips for Perfectly Leavened Cookies
Baking soda reacts immediately with acidic ingredients, producing carbon dioxide that creates a quick rise and chewy texture in cookies, while baking powder contains both acid and base, offering a slower, double-acting leavening effect for a lighter, more tender crumb. Expert tips recommend using baking soda when recipes include natural acids like brown sugar or buttermilk to enhance browning and flavor, and opting for baking powder in recipes without acidic components to ensure consistent rise. Precise measurement and proper mixing of leavening agents are crucial for achieving perfectly leavened cookies with balanced texture and optimal spread.
Baking soda vs Baking powder for cookie leavening Infographic
 cookingyes.com
cookingyes.com