Rock salt lowers the freezing point of water, allowing ice cream to freeze faster and achieve a smoother texture by absorbing heat more efficiently than ice alone. Using rock salt in the freezing technique creates a slush mixture that maintains a consistent, colder temperature, which prevents large ice crystals from forming. This method is preferred over plain ice because it enhances the creaminess and overall quality of homemade ice cream, especially in pet-friendly recipes.
Table of Comparison
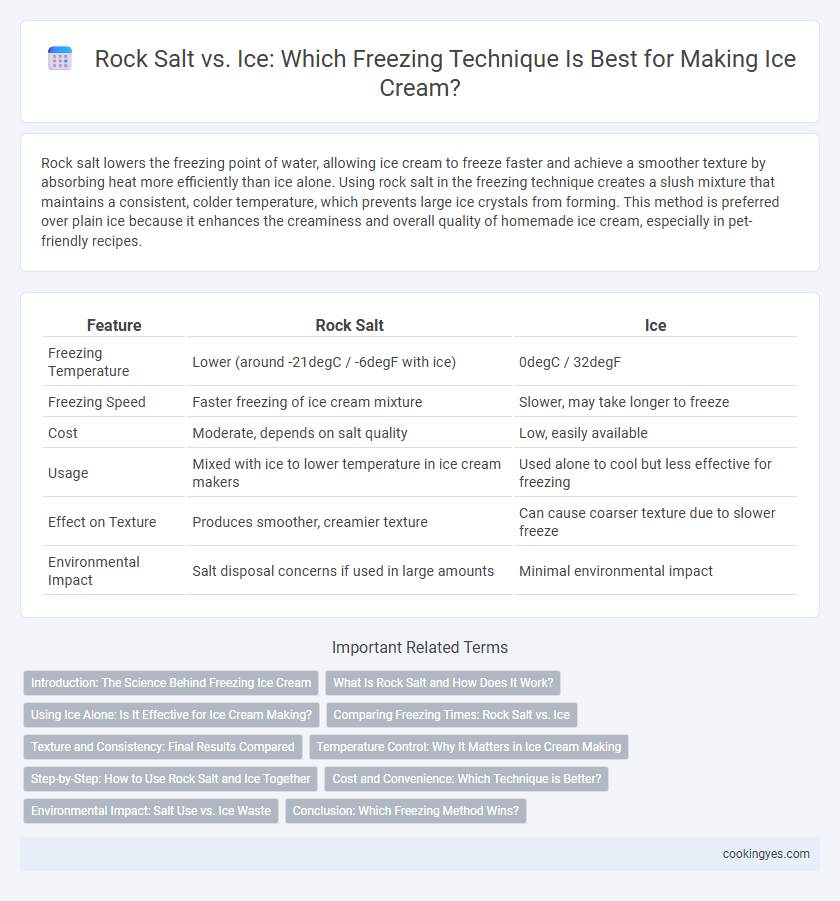
| Feature | Rock Salt | Ice |
|---|---|---|
| Freezing Temperature | Lower (around -21degC / -6degF with ice) | 0degC / 32degF |
| Freezing Speed | Faster freezing of ice cream mixture | Slower, may take longer to freeze |
| Cost | Moderate, depends on salt quality | Low, easily available |
| Usage | Mixed with ice to lower temperature in ice cream makers | Used alone to cool but less effective for freezing |
| Effect on Texture | Produces smoother, creamier texture | Can cause coarser texture due to slower freeze |
| Environmental Impact | Salt disposal concerns if used in large amounts | Minimal environmental impact |
Introduction: The Science Behind Freezing Ice Cream
Rock salt lowers the melting point of ice, creating a colder environment essential for efficiently freezing ice cream mixtures. This freezing technique accelerates ice crystallization, resulting in smoother texture and creamier consistency. Using rock salt instead of ice alone enhances thermal conductivity, optimizing the time and quality of the ice cream freezing process.
What Is Rock Salt and How Does It Work?
Rock salt, a coarse crystalline form of sodium chloride, lowers the freezing point of water when mixed with ice, creating an ultra-cold environment ideal for ice cream freezing. By disrupting ice formation, rock salt causes the ice to melt at a lower temperature, absorbing heat from the ice cream mixture quickly and enhancing the freezing process. This technique enables smooth, creamy texture development by preventing large ice crystals from forming during ice cream churning.
Using Ice Alone: Is It Effective for Ice Cream Making?
Using ice alone for freezing ice cream is less effective because it cannot reach temperatures low enough to rapidly freeze the mixture, resulting in a coarser texture. Rock salt lowers the freezing point of ice, enabling a colder environment that promotes smoother ice cream consistency. Without rock salt, ice melts faster and the freezing process slows, compromising the quality and creaminess of the final product.
Comparing Freezing Times: Rock Salt vs. Ice
Rock salt lowers the freezing point of ice, accelerating the freezing process and reducing ice cream freezing time significantly compared to using ice alone. Typically, rock salt ice mixtures can freeze ice cream in about 20-30 minutes, whereas ice-only methods often take 40-60 minutes due to slower heat extraction. This efficiency makes rock salt the preferred choice for faster and smoother ice cream texture development.
Texture and Consistency: Final Results Compared
Rock salt lowers the freezing point more effectively than ice, resulting in faster ice cream freezing and a smoother texture with fewer ice crystals. Ice alone freezes the mixture more slowly, often producing a grainier consistency due to larger ice crystals. Using rock salt in the freezing process ensures a creamier, more consistent final ice cream texture.
Temperature Control: Why It Matters in Ice Cream Making
Rock salt lowers the freezing point of ice, enabling a more consistent and lower temperature crucial for smooth ice cream texture. Precise temperature control prevents large ice crystals from forming, ensuring creamy, high-quality ice cream. Using rock salt instead of plain ice accelerates the freezing process, enhancing the overall texture and flavor stability.
Step-by-Step: How to Use Rock Salt and Ice Together
To freeze ice cream effectively using rock salt and ice, layer ice cubes in a large container, then sprinkle a generous amount of rock salt over the ice to lower the freezing point and accelerate cooling. Place the ice cream mixture in a smaller sealed container and submerge it into the salted ice, ensuring even contact for consistent freezing. Stirring the ice cream intermittently during the process helps achieve a smooth texture by preventing large ice crystals from forming.
Cost and Convenience: Which Technique is Better?
Rock salt is more cost-effective than ice for freezing ice cream because it lowers the freezing point, allowing faster and more consistent cooling with less quantity needed. Ice is convenient but requires larger volumes and frequent replenishment, increasing hassle and overall expense. For efficient and budget-friendly homemade ice cream preparation, rock salt is the superior freezing technique.
Environmental Impact: Salt Use vs. Ice Waste
Rock salt accelerates the freezing process by lowering the melting point of ice, reducing overall ice consumption but introducing saline runoff that can harm soil and water ecosystems. Using only ice avoids chemical discharge and potential environmental contamination but leads to greater ice waste and higher energy demands for ice production. Balancing these factors is crucial in selecting an eco-friendly freezing technique in ice cream preparation.
Conclusion: Which Freezing Method Wins?
Rock salt lowers the freezing point of ice, allowing the mixture to reach temperatures around -21degC (-6degF), which freezes ice cream faster and creates a smoother texture by preventing large ice crystals. Ice alone maintains 0degC (32degF), resulting in slower freezing and coarser texture. For optimal ice cream consistency and efficiency, the rock salt and ice combination remains the superior freezing method.
Rock Salt vs Ice for Freezing Technique Infographic
 cookingyes.com
cookingyes.com