Baking soda and baking powder both serve as leavening agents in cake recipes, but they function differently depending on the acidity of the batter. Baking soda requires an acidic ingredient like buttermilk or lemon juice to activate and produce carbon dioxide, which helps the cake rise. Baking powder contains both an acid and a base, allowing it to react twice--once when mixed with wet ingredients and again when heated--providing consistent leavening without the need for additional acidic components.
Table of Comparison
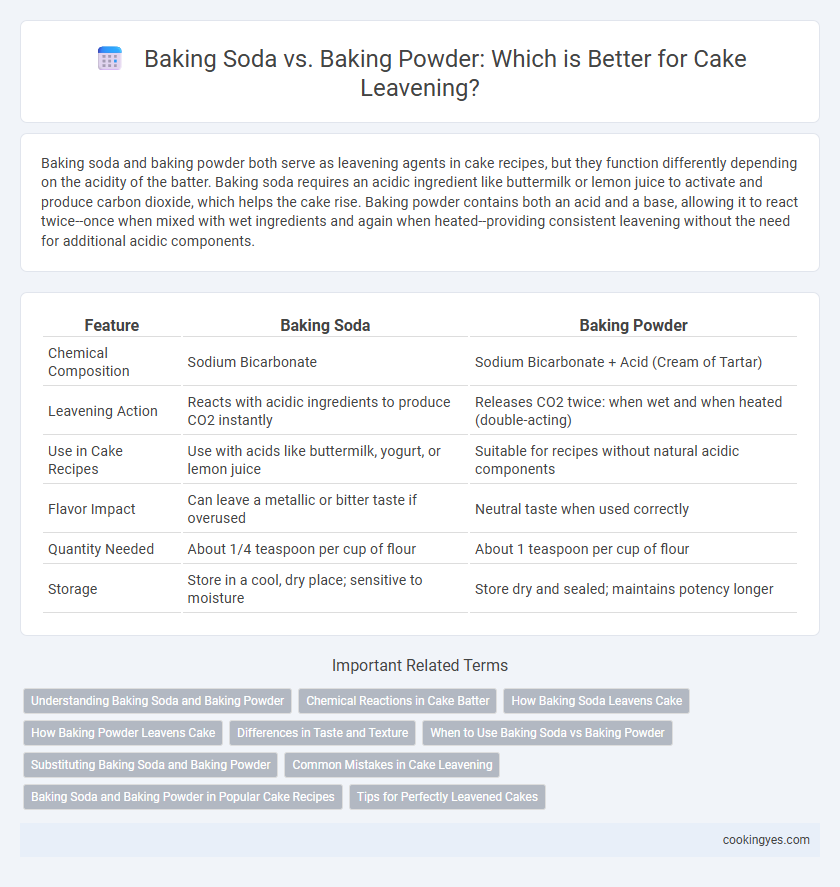
| Feature | Baking Soda | Baking Powder |
|---|---|---|
| Chemical Composition | Sodium Bicarbonate | Sodium Bicarbonate + Acid (Cream of Tartar) |
| Leavening Action | Reacts with acidic ingredients to produce CO2 instantly | Releases CO2 twice: when wet and when heated (double-acting) |
| Use in Cake Recipes | Use with acids like buttermilk, yogurt, or lemon juice | Suitable for recipes without natural acidic components |
| Flavor Impact | Can leave a metallic or bitter taste if overused | Neutral taste when used correctly |
| Quantity Needed | About 1/4 teaspoon per cup of flour | About 1 teaspoon per cup of flour |
| Storage | Store in a cool, dry place; sensitive to moisture | Store dry and sealed; maintains potency longer |
Understanding Baking Soda and Baking Powder
Baking soda is a pure chemical leavening agent, sodium bicarbonate, that requires an acidic ingredient like buttermilk or lemon juice to activate and produce carbon dioxide for cake rise. Baking powder contains sodium bicarbonate and an acidifying agent, typically cream of tartar, along with a drying agent, enabling it to react twice: once when wet and again during baking. Understanding the distinct chemical properties and activation mechanisms of baking soda and baking powder is essential for achieving optimal cake texture and proper leavening.
Chemical Reactions in Cake Batter
Baking soda reacts with acidic ingredients in cake batter, producing carbon dioxide gas that creates bubbles and causes the cake to rise. Baking powder contains both an acid and a base, triggering a double chemical reaction: one when mixed with wet ingredients and another when exposed to heat, ensuring consistent leavening. Understanding these chemical reactions helps bakers optimize texture and volume in cake preparation.
How Baking Soda Leavens Cake
Baking soda leavens cake through a chemical reaction with acidic ingredients, releasing carbon dioxide gas that forms bubbles and causes the batter to rise. This alkaline compound requires an acid, such as buttermilk, yogurt, or vinegar, to activate its leavening properties effectively. Proper balance ensures a light, airy texture and prevents metallic or soapy aftertaste in the final cake.
How Baking Powder Leavens Cake
Baking powder leavens cake by releasing carbon dioxide gas when it reacts with moisture and heat, creating bubbles that expand and cause the batter to rise. It contains both an acid and a base, enabling it to produce lift without additional acidic ingredients in the recipe. This dual-action ensures consistent and reliable rising, resulting in a light and fluffy cake texture.
Differences in Taste and Texture
Baking soda produces a coarser crumb and a slightly metallic or soapy aftertaste if not balanced with acidic ingredients, while baking powder yields a tender, fluffy texture with neutral flavor due to its balanced acid-base composition. Cakes made with baking soda often have a denser structure and browner color because of Maillard reaction enhancement. Baking powder ensures consistent rise and lightness by providing controlled carbon dioxide release, resulting in a softer mouthfeel and mild sweetness in cake texture.
When to Use Baking Soda vs Baking Powder
Baking soda is ideal for recipes containing acidic ingredients like yogurt, lemon juice, or buttermilk, as it reacts immediately to produce carbon dioxide, causing the cake to rise. Baking powder, which contains both an acid and a base, is suitable for recipes without acidic components because it activates twice--once when wet and again when heated--ensuring consistent leavening. Use baking soda for quick reactions in acidic batters and baking powder for a balanced rise in neutral or non-acidic cake mixtures.
Substituting Baking Soda and Baking Powder
Baking soda and baking powder both serve as leavening agents in cake baking but are not directly interchangeable due to their chemical properties. When substituting baking powder for baking soda, use approximately three times the amount because baking powder contains additional acidic components necessary for activation. Conversely, when replacing baking powder with baking soda, include an acidic ingredient such as cream of tartar or lemon juice to ensure proper leavening and avoid a metallic aftertaste.
Common Mistakes in Cake Leavening
Using baking soda instead of baking powder can cause cakes to have a metallic taste or an uneven rise due to improper neutralization of acids in the batter. A common mistake is not adjusting the acidic ingredients when substituting one leavening agent for the other, which leads to dense or flat cakes. Overmeasuring either baking soda or baking powder results in a bitter flavor and an undesirable crumb structure in the finished cake.
Baking Soda and Baking Powder in Popular Cake Recipes
Baking soda, a pure chemical leavening agent, requires an acidic ingredient like buttermilk or yogurt to activate and produce carbon dioxide, which helps cakes rise. Baking powder contains both an acid and a base, enabling it to leaven cakes independently in recipes without additional acidic components. Popular cake recipes often use baking powder for a balanced rise and tender crumb, while baking soda is favored in recipes with natural acids for a strong, quick lift and enhanced browning.
Tips for Perfectly Leavened Cakes
Use baking soda when your cake batter contains acidic ingredients like buttermilk or lemon juice to activate proper leavening. Baking powder is ideal for recipes without natural acids, ensuring consistent rise and texture. Measure accurately and avoid overmixing to maintain optimal crumb and tenderness in your cake.
Baking Soda vs Baking Powder for cake leavening Infographic
 cookingyes.com
cookingyes.com